Introduction
Post-transplant lymphoproliferative disorders (PTLD) are rare and severe complications of organ or allogeneic stem cell transplants (AST), often linked to EBV. The recommended first-line management of systemic PTLD, in addition to lowering immunosuppression, currently consists of response-tailored treatment (RTT): monotherapy with rituximab (R) and, in case of CR, a continuation of rituximab as monotherapy (RM), otherwise 4 courses of R-CHOP. The data in the literature are limited to a few very rare prospective studies and limited series, generally monocentric.
Patients and methods
The French national K-virogref database, supported by the French Institute against Cancer (INCa), started in September 2013, gathers data from PTLDs confirmed by biopsy and re-reading in the Lymphopath network. Data from 10 years of this registry have been analyzed.
Results
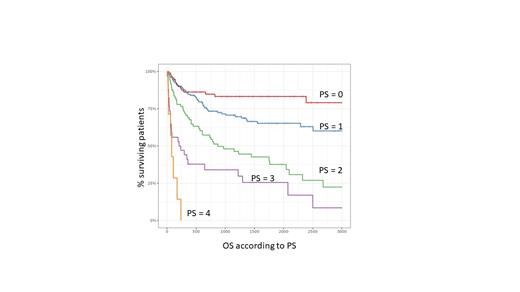
A total of 525 adult patients are analyzed, the median age of transplant is 44 years, that of diagnosis of PTLD 58 years, 63% are men. Transplants are 61% kidneys, 17% livers, 6% lungs, 6% hearts, 8% stem cells. The median time from transplant to diagnosis of PTLD is 97 months, with 16% of diagnosis within the first year. The histology is of early lesion type in 4.6%, polymorphic in 12% and of lymphoma type in 83.3%, 46.7% are linked to EBV. Of the total 435 are systemic and 90 (17%) are localized to the central nervous system (CNS); predictors of CNS PTLD are later age at transplant (52 years vs 43 years), male gender ( p=1.10-3), a kidney transplant (69% vs 60%), CNS PTLDs are more often late after the transplant, more often of the lymphoma type and almost always linked to EBV (97.8%). The EBV status of the recipient at the time of transplantation was known in 242 cases, of which 50 were EBV negative; the negative EBV status is linked to an earlier age at diagnosis (43 years vs 58 years), a shorter time between transplant and diagnosis (11.5 months vs 66.5), more often early forms, more frequent localization in the graft (24% vs 9%), better survival. The median overall survival (OS) of all patients is 2700 days, tumor EBV status has no influence on OS, progression-free survival (PFS) is 1500 days. Median survivals are 2600 days for AST, 4600 for hearts, 5400 for lungs and not reached (NR) for livers and kidneys. Among the classic prognostic criteria, the IPI is not discriminating between 0 and 2, whereas the personal status is clearly discriminating (cf figure). Of the patients CD20+ without CNS localization nor Burkitt, treatment data were known for 274 patients: 27% received only immuno-chemotherapy (IC), 38% had RM and 35% R then R-CHOP. Survival is identical between IC and R then R-CHOP, with less chemotherapy in the sequential treatment, and is significantly higher (p=0.011) for RM, the RTT has a trend to a better OS than IC (p=0.062). In the event of chemotherapy, the use of an anthracycline has a better OS (median NR vs 750 days).
Conclusion
To our knowledge, this is the largest series of PTLD presented to date. The results of OS are similar to those presented in the prospective studies with selected patients and our data confirm the interest of RTT, with in case of chemotherapy, the need to use an anthracycline. The most discriminating and simplest prognostic criterion is PS. However, this very large series only concerns adult patients and cannot describe real-life pediatric PTLD.
Disclosures
Roulin:Kitegilead: Other: Conference speaker and travel grants, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Scientific advisory board; BMS: Consultancy, Other: Conference speaker, Speakers Bureau. Azar:Sanofi: Consultancy, Honoraria; Janssen: Consultancy. Cluzeau:AbbVie: Consultancy, Other: support attending meetings/travel; Jazz Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: support attending meetings/travel; Agios: Consultancy; Servier: Consultancy, Honoraria, Other: support attending meetings/travel; BluePrint: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: support attending meetings/travel; Astellas: Honoraria; Incyte: Honoraria; Pfizer: Other: support attending meetings/travel. Bachy:Novartis: Honoraria, Other: Personal Fees; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Takeda: Honoraria; Incyte: Honoraria; Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Amgen: Research Funding; Kite, a Gilead Company: Honoraria, Other: Personal Fees; Roche: Consultancy, Honoraria. Jardin:Janssen, Gilead, AbbVie, F. Hoffmann-La Roche Ltd, BMS, Takeda: Honoraria. Ysebaert:Abbvie: Honoraria, Research Funding, Speakers Bureau; Beigene: Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Gilead/Kite: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria. Choquet:Atara: Consultancy; Pierre Fabre: Consultancy; Novartis: Consultancy; Gilead kite: Consultancy; Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal